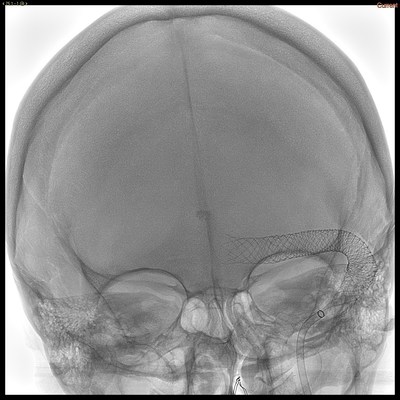
LAKE FOREST, Calif., Dec. 16, 2022 /PRNewswire/ — Sonorous NV, Inc. Chairman, Dave Ferrera, announced treatment of the first patients in North America with the BossStent® device designed to treat patients with symptomatic cerebral venous diseases. The single-use, disposable device is an endoluminal device that is placed into a cerebral venous sinus stenosis to normalize blood flow and pressure gradients causing pulsatile tinnitus and/or IIH (idiopathic intracranial hypertension), etc. The initial cases in North America were performed by Dr. Vitor Mendes Pereira, Neurosurgeon and the Director of Endovascular Research and Innovation at the St. Michael’s Hospital. Said Dr. Mendes Pereira, “The BossStent® Sonorous NV device produced excellent clinical results. It offers a breakthrough solution for patients with symptomatic cerebral venous diseases associated with venous sinus stenosis who require interventional therapy. Knowing such a solution exists places both patients and physicians at ease, and will create opportunities in the future for patients who are experiencing these debilitating symptoms.”
Added Ferrera, “We are confident that use of the BossStent® device will provide an on-label solution for symptomatic cerebral venous diseases leading to papilledema, pulsatile tinnitus and IIH. Over time, we believe use of the device will become the standard of care.”
Sonorous NV received its initial seed funding in Q1 of 2022 and quickly developed their product to achieve first clinical use in less than 1 year. Both the EU and FDA clinical trials will be initiated in Q2 2023 for the purpose of obtaining European and US regulatory approvals respectively in Q1 2024.
Contact Information:
Dave Ferrera – Chairman
Sonorous NV, Inc.
Lake Forest, CA USA
Email: 350793@email4pr.com
Phone: +1-310-614-0636
Web: www.sonorousnv.com
SOURCE Sonorous NV