XI’AN, China, Jan. 11, 2022 /PRNewswire/ — As the cost of branded drugs keeps increasing, challenging the purchasing power of patients, the demand for generic medicines is on the rise. With the same active ingredients and intended use as the branded ones, generic medicines are relatively much cheaper as the manufacturers do not need to develop or market them as new drugs. Nevertheless, they are required to meet the same quality standards as the branded drugs.
In China, the pharmaceutical industry landscape is undergoing a rapid evolution. The country has emerged as one of the largest generic drug markets in the world, warranting new regulations to evaluate generic drug consistency and enhance its international competitiveness. However, the National Evaluation Sampling and Test Project (NESTP), China’s existing domestic generic drug evaluation information source, fell short of meeting the fast-changing drug market requirement. Put simply, drugs imported from the EU and USA are typically manufactured through production processes and process controls different from those for domestic drugs and, therefore, cannot be suitably evaluated by NESTP.
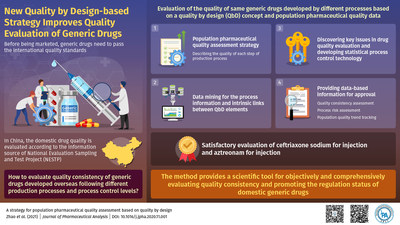
To bridge this gap, researchers from China came up with an innovative strategy to enable the quality evaluation of generic drugs developed by different processes. The study, led by Professor Changqin Hu of the National Institute for Food and Drug Control, and Professor Xiaomei Ling of Peking University, was published in Journal of Pharmaceutical Analysis. This paper was made available online on 4th November, 2020 and was published in Volume 11 Issue 5 of the journal on 31st October 2021.
The researchers designed their study aligning with quality standards of current Good Manufacturing Practice that stressed the "QbD" concept instead of the traditional "quality by test" approach. Prof. Hu explains, "According to QbD concept, quality of drugs can be maintained by ensuring the quality during each step of the design, development and manufacturing process. To develop an evaluation strategy that would assess the commonalities among different processes of generic drug development, we aimed to define universal indicators and methods to characterize different processes used for the same pharmaceutical product."
Fortunately, information related to the drug development processes was documented and available in NESTP and other literature. Based on this information, the researchers developed an evaluation process called "population pharmaceutical quality assessment" for mining the process information related to sample-population quality and investigating intrinsic links between QbD elements. Prof. Ling elaborates, "Our method provided a scientific tool for objectively and comprehensively evaluating quality consistency and promoting the regulation status of domestic generic drugs."
To validate their new strategy, the researchers performed quality consistency assessment of generic ceftriaxone sodium injections and process risk assessment and population quality trend tracking of generic aztreonam injections. Both assessments offered satisfactory results. "The new method is an effective and economical means to improve product quality by discovering key issues in drug quality evaluation through data mining. Moreover, it would likely facilitate timely prediction of various hidden but avoidable quality hazards, serving the regulatory perspective," Dr. Zhao surmises.
The researchers are hopeful that by leveraging the continuous addition of data in the knowledge base, they can improve their strategy further for superior decision-making regarding drug regulation.
Reference
Title of original paper: A strategy for population pharmaceutical quality assessment based on quality by design
Journal: Journal of Pharmaceutical Analysis
DOI: https://doi.org/10.1016/j.jpha.2020.11.001
Your Press Release Source
Journal of Pharmaceutical Analysis
Media Contact:
Fen Qiu
Xi’an, China
327243@email4pr.com
+86-131-5206-8068
SOURCE Journal of Pharmaceutical Analysis