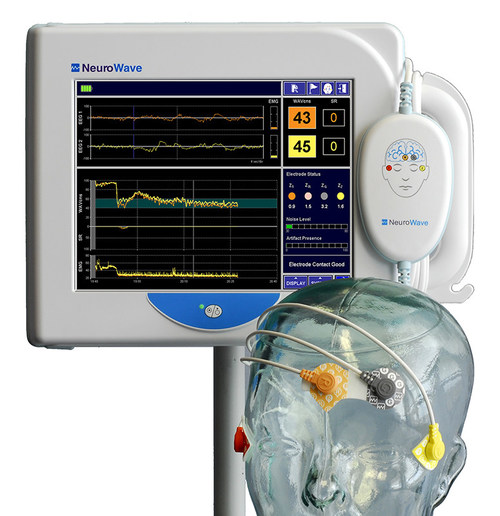
BEACHWOOD, Ohio, Sept. 29, 2021 /PRNewswire/ — NeuroWave Systems Inc. is pleased to announce the FDA clearance of its NeuroSENSE® NS-901 Monitor – a new generation brain function monitor for assessing the adequacy of anesthesia and sedation in clinical settings. NeuroSENSE aids clinicians in monitoring the state of the brain in anesthetized patients (18 years of age and older). The system acquires bilateral EEG signals and calculates bilateral WAVCNS indices, one for each brain hemisphere. The WAVCNS (Wavelet Anesthetic Value for Central Nervous System) is a proprietary processed EEG parameter that quantifies the hypnotic effect of anesthetics on the brain. Powered by wavelet-based leading-edge signal processing technologies tailored to EEG signals, NeuroSENSE provides immediate and delay-free tracking of changes in patient state. The monitor also provides a high-resolution bilateral density spectral array and bilateral suppression ratios.
NeuroSENSE is a unique bilateral brain function monitor for anesthesia specialists that simultaneously provides independent indices of anesthetic effect for each side of the brain. This offers greater insight into the brain of anesthetized or sedated patients and assists clinicians in detecting brain function asymmetry (due to, for example, unilateral pathology) and in making optimal decisions for safer and improved patient care.
"Our NeuroSENSE monitors have successfully been used in Europe over the last decade. We are particularly proud and excited for the opportunity to bring this technology to the domestic US market and to continue leveraging its advances for our closed-loop total intravenous anesthesia system," commented Ms. Tatjana Zikov, the President of NeuroWave Systems Inc. She added: "This next generation brain function monitor offers unique features to this space such as an instant response, individual anesthetic depth index for each brain side, linear response to increasingly deep anesthesia, and superior discrimination between consciousness and unconsciousness."
Dr. Stephane Bibian, NeuroWave’s Vice President, Engineering and Clinical Research, further commented: "We are deeply committed to innovation in anesthesia care, and this FDA clearance is a cornerstone towards our overarching goal of automating anesthesia drug delivery. I would like to thank the University of British Columbia (UBC) for helping develop and validate the NeuroSENSE technology. We eagerly continue the development of our closed-loop anesthesia system integrating NeuroSENSE and UBC’s clinically validated control algorithms and are grateful for the continued support from the U.S. Department of Defense[1] and National Institutes of Health[2]. Our current focus is the development and regulatory clearance of a 2–channel infusion pump system under the administration by the Naval Medical Research Center’s (NMRC) Naval Advanced Medical Development (NAMD)[3]. Our vision is to vertically integrate all these technologies into a pioneering anesthesia product."
"We are thrilled our team has achieved such an important milestone and I would like to thank each one of our employees for all their hard work and commitment. We expect that the unique performance features brought by NeuroSENSE will have significant positive impact on the brain function monitoring space. We look forward to partnering with hospitals and distributors to expand the use of our novel technology," concluded Mr. Robert N. Schmidt, NeuroWave’s Chairman.
For more information, visit www.NeuroWaveSystems.com.
Contact:
Dr. Brian Kolkowski, VP of Business Development, NeuroWave Systems Inc., 216-649-0376, or
319810@email4pr.com
.
[1] NeuroSENSE clinical validation study was funded by the U.S. Army Medical Research and Materiel Command under Contract no. W81XWH-06-C-0016, and the analysis of data acquired in this study was supported by the Office of Naval Research under Contract no. N00014-14-C-0324. Closed-loop anesthesia research mentioned in this press release was supported by the U.S. Army Medical Research and Materiel Command under Contract No. W81XWH-11-C-0078. The views, opinions and/or findings contained in this press release are those of the author(s) and should not be construed as an official Department of the Navy or Department of Defense position, policy or decision unless so designated by other documentation.
[2] Closed-loop anesthesia research mentioned in this press release when relating to pediatrics is supported by National Institute of General Medical Sciences of the National Institutes of Health under award number R43GM112358. The content of this press release is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
[3] The 2-channel infusion pump mentioned in this press release is supported by the U.S. Army Medical Research and Development Command under Contract No. W81XWH20C0115. The views, opinions and/or findings contained in this press release are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation.
SOURCE NeuroWave Systems Inc.