PHOENIX, Aug. 3, 2022 /PRNewswire/ — There is no therapeutic that has been shown to stop or reverse the devastating memory loss of Alzheimer’s Disease. That may have all changed today with publication of a scientific paper in the journal Medicines. The paper reports that a small group of Alzheimer’s patients given in-home treatment with a bioengineered head device actually maintained their level of cognitive functioning through 2.5 years of treatment.
Most Alzheimer’s patients would have suffered progressive memory loss over that long a period of time, but it did not happen to those given daily Transcranial Electromagnetic Treatment (TEMT) in this landmark study. Not only did they not show significant memory decline in all six clinical tasks administered over the 2.5-year period, but the Alzheimer’s patients’ own caregivers evaluated them as not having any further memory loss from when they started this lengthy study.
The company doing this TEMT research, NeuroEM Therapeutics, had previously published a study in the Journal of Alzheimer’s Disease reporting that daily TEMT for a 2-month period reversed the cognitive impairment in patients with Alzheimer’s Disease.
In 2020, NeuroEM Therapeutics was given the FDA’s first "Breakthrough Designation" for an Alzheimer’s therapeutic (TEMT) and is now being guided by the FDA in its TEMT clinical studies.
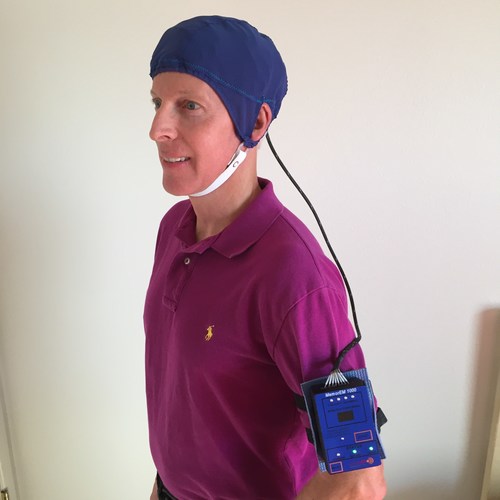
In the present study, Alzheimer’s patients received daily in-home TEMT for 18 months during a 31-month period with the company’s proprietary "MemorEM" device. "Since these patients only received TEMT for 18 of the 31 months, one wonders if perhaps daily TEMT for all 31 months could have provided the same memory reversal as in our earlier study," said Dr. Gary Arendash, the study’s principle investigator and CEO of NeuroEM Therapeutics.
The device contains eight electromagnetic emitters embedded in a soft two-layered cap that together provide full brain treatment. This is important because Alzheimer’s Disease affects many areas of the brain.
Aside from stopping memory impairment, TEMT also affected multiple markers of Alzheimer’s Disease in the brain by inducing long-term reductions in p-tau, C-reactive protein, A-beta1-40, and A-beta1-42.
Long-term TEMT administration by the patients’ caregivers was completely safe, with no undesirable side effects. Moreover, the MemorEM device administering TEMT allows near complete patient mobility.
"Drugs have not been effective against AD because they don’t get inside brain cells (neurons) well and don’t effectively target the primary causes of Alzheimer’s Disease, which appear to be toxic protein aggregation, low energy (ATP) production, and a dysfunctional immune system. TEMT targets all three of these likely contributors to Alzheimer’s memory decline," stated Dr. Arendash.
The study concluded that "Although only a limited number of Alzheimer’s patients were involved in this study, the results suggest that TEMT can stop the cognitive decline of Alzheimer’s Disease over a period of at least 2.5 years, and do so with no safety issues."
This is breakthrough news for the 6.2 million Alzheimer’s patients and their families in the U.S., especially given so many Alzheimer’s drugs have failed over the last two decades. NeuroEM Therapeutics anticipates starting a Pivotal clinical trial later this year, the results of which it hopes will form the basis for FDA approval of TEMT to treat Alzheimer’s Disease.
NeuroEM is a privately-held clinical-stage biotech company focused on the clinical development of its proprietary Transcranial Electromagnetic Treatment (TEMT) technology against Alzheimer’s Disease.
INFORMATION CONTACT:
Dr. Gary Arendash
341720@email4pr.com
Tel: (480) 395-1481
New Study Reference:
"Transcranial Electromagnetic Treatment Stops Alzheimer’s Disease Cognitive Decline Over a 2½ Year Period: A Pilot Study" published August 3rd, 2022 online in Medicines.
SOURCE NeuroEM Therapeutics