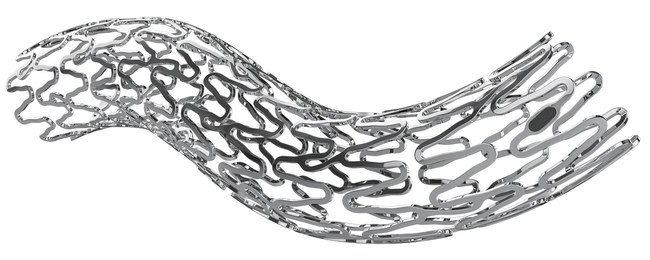
WINSEN, Germany, May 24, 2021 /PRNewswire/ — amg International GmbH (“amg” or the “Company”), a wholly owned subsidiary of Dublin, Ireland based Q3 Medical Devices Limited (Q3), further establishes its leadership role in the fast-paced development of novel biodegradable implants. The Company announced that it has received CE Mark approval for its second fully biodegradable product, the UNITY-B balloon expandable biodegradable biliary stent (BEBS) for endoscopic use. The UNITY-B will compliment ARCHIMEDES™, the world’s first CE approved pancreaticobiliary biodegradable implant.
The UNITY-B Biodegradable implant was developed as a complimentary biodegradable metallic stent to amg’s ARCHIMEDES biodegradable pancreaticobiliary stent, which is currently available in fast, medium, and long-lasting degradation profiles. ARCHIMEDES obtained CE mark approval in 2018 and was subsequently launched through a global distribution partnership with Medtronic. Before the introduction of amg’s biodegradable biliary and pancreatic portfolio, biliary and pancreatic duct implants were made of non-degrading plastics or metals, which typically required multiple procedures to place the stents, treat complications, and ultimately retrieve or replace them as their removal is mandatory in most cases and adds significant total cost to the procedures.
The UNITY-B biodegradable stent is a large diameter balloon expandable implant that is introduced endoscopically and is intended to facilitate the drainage of obstructed ducts requiring larger openings. The UNITY-B biodegradable stent is designed to replace the estimated ¤1 billion+ global market for metal biliary implants. Much like the ARCHIMEDES, the UNITY-B biodegradable stent was developed as an alternative to avoid the second removal procedures required with the traditional fully and partially covered self-expanding metallic stents. The amg biodegradable portfolio provides an advanced treatment option to the traditional plastic and metallic implants used by Gastroenterologists, Surgeons, and Interventional Radiologist to reduce the total cost of care and complications associated with the older generation of non-biodegradable plastic and metallic implants.
“The CE mark approval of the UNITY-B stent represents yet another milestone for amg International and all the companies of Q3 Medical and our mission to ‘Create Value By Helping People’. In these uncertain times, being able to reduce the total cost of care using advanced, clinically proven products is more important than ever,” said Eric K. Mangiardi, CEO of Q3. “As we continue the development of additional biodegradable technologies to expand our portfolio for use in the gastrointestinal tract and for the vasculature, we are striving to shift the paradigm for treatments by reducing complications and eliminating the need for additional removal procedures associated with the current treatment options.
“The approval of the UNITY-B balloon-expandable biodegradable stent represents yet another major innovative breakthrough that provides my patients and I, a more optimal treatment solution for obstructed biliary ducts versus traditional non-degradable technology. These next-generation biodegradable stents can prove to be a game changer for endoscopic management of biliary obstruction. Additionally, with the current state of the global pandemic, biodegradable implants provide the opportunity to reduce both, the cost and avoidable exposure, through the elimination of the second removal procedure of conventional stents – thus lowering the overall burden to the healthcare systems around the world,” said Dr Sundeep Lakhtakia of the Asian Institute of Gastroenterology in Hyderabad, India.
In a recent safety and efficacy clinical study conducted, the UNITY-B balloon expandable biodegradable biliary stent system showed no (0) Adverse Events and no (0) Serious Adverse Events reported as related or even possibly related to the UNITY-B study device. “The final clinical success evaluation resulted to a success rate of 94.4% which is outstanding in comparison to the ESGE 2012 guideline for traditional products, which just for stent dysfunction already reports 41%, 27% and 20% failure rate for plastic stents, uncovered SEMS (self-expandable metallic stents) and covered SEMS, respectively” stated Dr. Hairol A. Othman. Further procedural success was almost 100% compared to the ESGE 2018 guideline which reports that ERCP procedures fail in 10% – 20% of patients. Dr. Hairol A. Othman and colleagues of Sunway Medical Centre, Kuala Lumpur, Malayasia further stated, “the result from our multi-center clinical study is very encouraging.”
“This is positive news, as this new Biodegradable toolkit of the UNITY-B balloon expandable biodegradable stent (BEBS) replacement for metal stents coupled with the ARCHIMEDES biodegradable replacement for plastic stents gives us the potential to reduce complication rates and procedural costs for removal typically associated with the older technology. In addition, the advancements can also be expanded to other indications in the gastrointestinal tract like the esophagus, colon, and pseudocyst drainage applications further expanding its utility and benefits,” noted Dr. Paul Yeaton, Chief of Gastroenterology at the Carilion Clinic in Roanoke, VA.
To see more about the UNITY-B balloon expandable biodegradable stent please review the links below:
Bilateral Balloon Expandable Biodegradable Stent for Postcholecystectomy Perihilar Biliary Stricture – VideoGIE:
https://www.videogie.org/article/S2468-4481(20)30320-9/fulltext
https://www.videogie.org/cms/10.1016/j.vgie.2020.11.001/attachment/40cc4794-8acd-4653-b3a6-cc2afd81eed7/mmc1.mp4
About amg International GmbH
amg International GmbH, part of the Q3 Medical Devices Limited family of companies, was established in 1997, yet our manufacturing partner has over 20 years of experience in the design and development of medical devices.
amg has recently shifted its focus to its AMG Gastro line of advanced Biodegradable gastroenterological products. Utilizing proprietary processes and materials to develop the world’s first bioresorbable polymer and metal stent technologies.
Developed and manufactured in Germany, providing innovative and quality products ultimately to the patients who need them is our number one priority.
About Q3 Medical Devices Ltd.
Q3 Medical Devices Ltd. is an Ireland based holding company with multiple global operations in Germany, China, & the United States along with a strong global partnerships, and an ever growing strategic investor base. The holding and its companies are focused on the development, manufacturing and distribution of its novel bioresorbable, micro invasive, localized intraluminal drug delivery, and core products platforms for interventional cardiology, peripheral vascular, and non-vascular diseases.
Q3 Medical Devices Ltd. was formed by a global group of entrepreneurs, manufactures, distributors, industry doctors, and investors, focused on the development and acquisition of medical device businesses with annual revenues between 1-10 Million. The acquisitions are targeted in areas that expand the groups manufacturing base and capabilities, grow its distribution channel and accelerate its products offering, focusing on the minimally invasive treatment of patients with cardiology, peripheral vascular and non-vascular diseases.
Forward Looking Statements
This announcement includes “forward-looking statements” which incorporate all statements other than statements of historical facts, including, without limitation, those regarding the Group’s financial position, business strategy, plans and objectives of management for future operations (including development plans and objectives relating to the Group’s products and services), and any statements preceded by, followed by or that include forward-looking terminology such as the words “targets”, “believes”, “estimates”, “expects”, “aims”, “intends”, “will”, “can”, “may”, “anticipates”, “would”, “should”, “could” or similar expressions or the negative thereof. Such forward-looking statements involve known and unknown risks, uncertainties and other important factors beyond the Group’s control that could cause the actual results, performance or achievements of the Group to be materially different from future results, performance or achievements expressed or implied by such forward-looking statements. Such forward-looking statements are based on numerous assumptions regarding the Group´s present and future business strategies and the environment in which the Group will operate in the future. Among the important factors that could cause the Group’s actual results, performance or achievements to differ materially from those in forward-looking statements include those relating to Q3 Medical’s & QualiMed’s funding requirements, regulatory approvals, clinical trials, reliance on third parties, intellectual property, key personnel and other factors. These forward-looking statements are valid at the date of this announcement. The Group expressly disclaims any obligation or undertaking to disseminate any updates or revisions to any forward-looking statements contained in this announcement to reflect any change in the Group’s expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based. As a result of these factors, readers are cautioned not to rely on any forward-looking statement.
Novel Biodegradable Stent Technology Approved for Sale in EuropeMedia requests to:
Eric K. Mangiardi
President & CEO
Q3 Medical Devices Ltd.
310624@email4pr.com
+353 86 782 7296
SOURCE Q3 Medical Devices Ltd.